The Element Neon Neon is a chemical element in the periodic table that has the symbol Ne and atomic number 10. A colorless nearly inert noble gas, neon gives a distinct reddish glow when used in vacuum discharge tubes and neon lamps and is found in air in trace amounts. Although neon is the fourth most abundant element in the universe, only 0.0018% of the earth's atmosphere is neon. The largest use for neon gas is in advertising signs. Neon is also used to make high voltage indicators and is combined with helium to make helium-neon lasers. Liquid neon is used as a cryogenic refrigerant.

Neon On The Periodic Table

The Element Neon
[Click for Isotope Data]
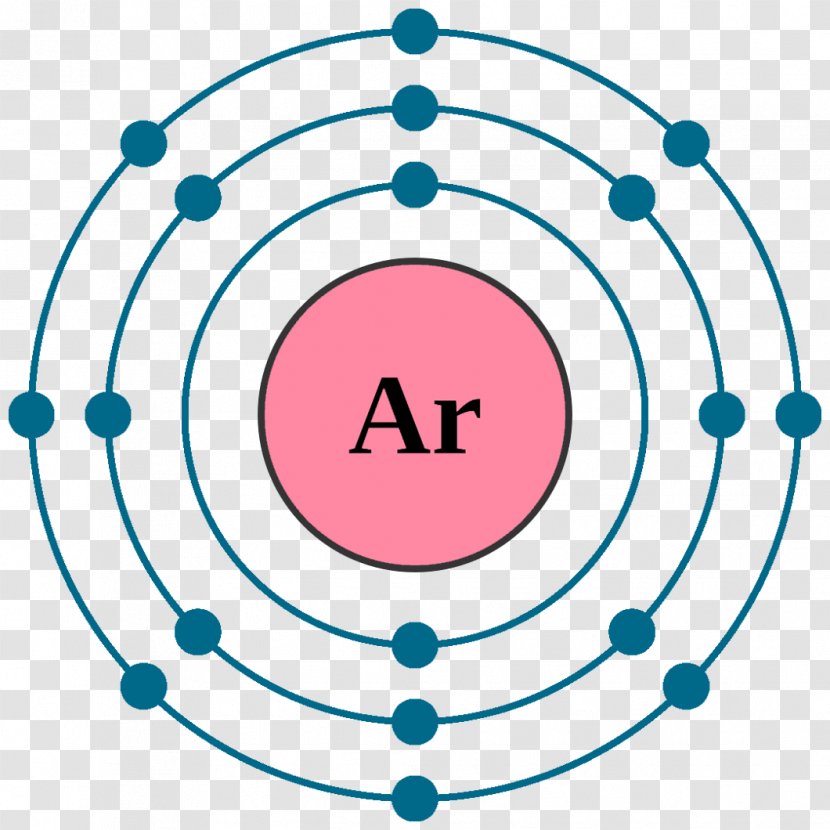
Neon Atom Diagram
Atomic Number: 10
Atomic Weight: 20.1797
Melting Point: 24.56 K (-248.59°C or -415.46°F)
Boiling Point: 27.07 K (-246.08°C or -410.94°F)
Density: 0.0008999 grams per cubic centimeter
Phase at Room Temperature: Gas
Element Classification: Non-metal
Remove adobe cs6 master collection mac file download free. Period Number: 2
Group Number: 18

Group Name: Noble Gas
What's in a name? From the Greek word for new, neos.
Say what? Neon is pronounced as NEE-on.
History and Uses:
Neon was discovered by Sir William Ramsay, a Scottish chemist, and Morris M. Travers, an English chemist, shortly after their discovery of the element krypton in 1898. Like krypton, neon was discovered through the study of liquefied air. Although neon is the fourth most abundant element in the universe, only 0.0018% of the earth's atmosphere is neon.
The largest use for neon gas is in advertising signs. Neon is also used to make high voltage indicators and is combined with helium to make helium-neon lasers. Liquid neon is used as a cryogenic refrigerant. Neon is highly inert and forms no known compounds, although there is some evidence that it could form a compound with fluorine.
Estimated Crustal Abundance: 5×10-3 milligrams per kilogram
Estimated Oceanic Abundance: 1.2×10-4 milligrams per liter
Number of Stable Isotopes: 3 (View all isotope data)

Ionization Energy: 21.565 eV
Oxidation States: 0
Electron Shell Configuration: | 1s2 |
2s2 2p6 |
For questions about this page, please contact Steve Gagnon.
